Dermatological Manifestations of IBD
Dermatological Manifestations of IBD
January 30, 2024
Introduction: Prevalence
The objectives of this presentation were to explore the range of dermatologic manifestations associated with inflammatory bowel disease (IBD), emphasize the importance of early recognition and interdisciplinary collaboration in patient management, and consider treatment options. Early identification and management of these conditions is important to optimize patient outcomes for both skin and bowel disease.1,2
The estimated prevalence of dermatologic extra-intestinal manifestations of IBD ranges from 10–15% depending on the nature of the manifestation. For example, a prospective study in 352 patients with IBD found erythema nodosum (EN) and pyoderma gangrenosum (PG) were the most common dermatologic manifestations (7.4% and 2.3%, respectively).3 Furthermore, with use of biologic agents, particularly anti-tumour necrosis factor (TNF) agents, has been associated with an increase in dermatologic manifestations such as psoriasiform and pustular eruptions.1
Dermatological Manifestations of IBD: Classification
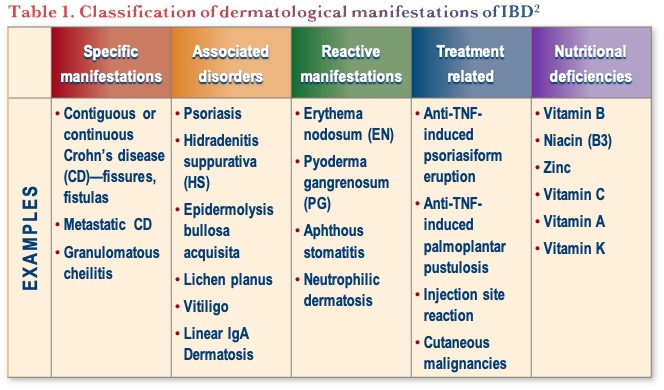
Dermatological manifestations can be classified based on the approach outlined in Table 1.
Dermatological Manifestations of IBD: Associated disorders
Psoriasis
A systematic review and meta-analysis (N=93 studies) found the prevalence of psoriasis in Crohn’s disease (CD) and ulcerative colitis (UC) to be 3.6% and 2.8%, respectively. The presence of CD or UC was also significantly associated with psoriasis, with an odds ratio (OR) of 2.0 (95% Confidence Interval [CI], 1.4–2.9 and OR 1.5; 95% CI, 1.2–2.0), respectively. Furthermore, psoriasis was significantly more common in IBD patients receiving anti-TNFs compared with those not receiving this treatment class, particularly among children with CD.4
Hidradenitis Suppurativa (HS)
A systematic review and meta-analysis included 8 studies with a total of 93,601 unique participants with HS showed 2.12-fold increased odds of having CD and 1.51-fold increased odds of having UC.5 Although traditionally treated with anti-TNFs, dermatologists are more recently moving to the use of IL-17 inhibitors for HS. However, the potential risk of unmasking IBD in a patient with subclinical disease must be kept in mind.
Dermatological Manifestations of IBD: Reactive manifestations
Erythema Nodosum and Pyoderma Gangrenosum
A cohort study from the Improve Care Now Network (ICN) database evaluated 32,497 pediatric patients and found an overall incidence of EN of 1.57% (95% CI, 1.43–1.71) and PG of 0.90% (95% CI, 0.80–1.00). Furthermore, worse intestinal disease (clinical assessment) was associated with both EN and PG and having EN or PG was significantly associated with having another extra-intestinal manifestation (EIM), such as arthritis or uveitis.6
In addition, a systematic review, and meta-analysis of 14 studies of patients with IBD was combined with 1,057 patients with IBD from the local institutional database. The incidence of PG ranged from 0.4 to 2.6% and was associated with female gender (Risk ratio [RR]: 1.328; 95% CI, 1.161–1.520), CD (RR: 1.193; 95% CI, 1.001–1.422), EN (RR: 9.281; 95% CI, 6.081–14.164), and ocular EIM (RR: 4.55; 95% CI, 3.04–6.81).7
Dermatological Manifestations of IBD: Treatment related
TNF-induced Psoriasiform Eruption
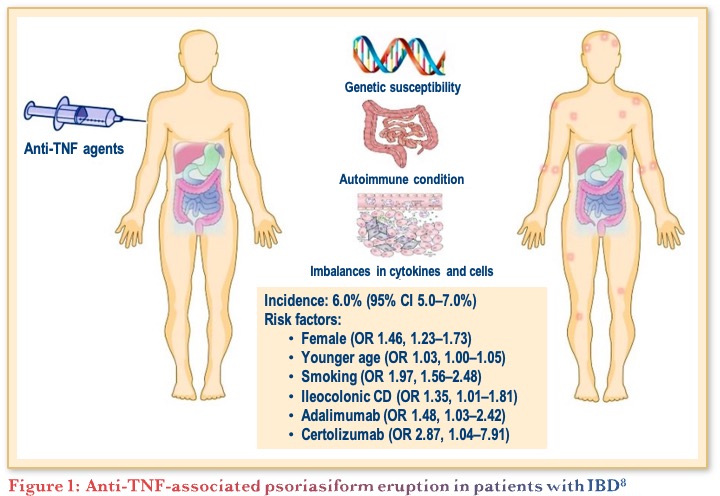
A systematic review and meta-analysis of 30 articles including 24,547 IBD patients treated by anti-TNF found an overall pooled incidence of psoriasis and/or psoriasiform lesions following anti-TNF therapy of 6.0%. Female gender, younger age, smoking, ileocolonic CD, and the type of anti-TNF were significantly associated with an increased risk as shown in Figure 1.8
Currently, there is no standard approach for the management of TNF inhibitor-induced psoriasis, but proposed algorithms recommend continuing anti-TNF for those with mild skin eruptions and controlled IBD. However, for those with uncontrolled IBD and a mild eruption or those with a moderate to severe skin eruption with controlled IBD, they recommend switching to a different anti-TNF. Lastly, for those with moderate to severe skin eruption and uncontrolled IBD they recommend changing class. It was noted that risankizumab and upadacitinib were not available when this algorithm was published.9 In these cases, targeting single cytokines is often not sufficient so dermatologists will consider switching to a broader immunomodulatory agent like a janus kinase (JAK) inhibitor.
Pediatric TNF-induced Psoriasiform Eruption
A multicentre retrospective chart review from 2000–2016 identified 103 pediatric patients who developed new onset psoriasiform eruption while taking an anti-TNF for a non-dermatologic condition. IBD was the most common indication in 91% of patients, and the primary non-dermatologic disease was under control in 92% of patients when they developed the psoriasiform eruption. Interestingly, of those that switched to a different anti-TNF, 31% developed a second psoriasiform eruption,10 supporting the approach of changing to a therapy with a different mechanism. Another study, a chart review of pediatric IBD patients treated with TNF inhibitors (N=638), found that 17% experienced a skin reaction. Following the skin reaction, 64% continued anti-TNF and 36% switched to ustekinumab. The children who switched to ustekinumab were more likely to have improved outcomes than those that stayed on anti-TNFs.11
Data are accumulating on the use of newer agents for EIMs in IBD. For example. a retrospective review of 111 patients (48% treated with ustekinumab, 88% had CD), found that the most common EIM was arthralgia (n=95/111, 84%). Clinical response of the EIM at week 52 was achieved in 36% of patients treated with ustekinumab (n=18/50) and 34% of patients (n=19/54) treated with vedolizumab, with no statistically significant difference (p=0.9).12
Dermatological Manifestations of IBD: Nutritional malabsorption
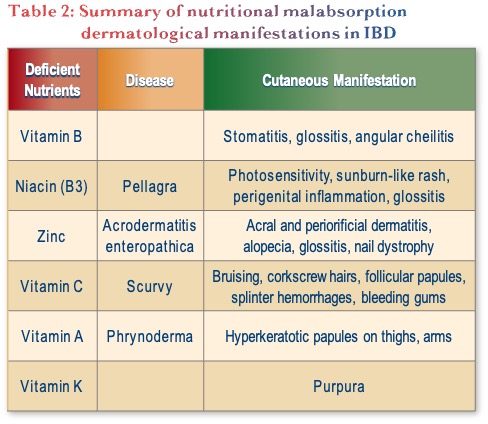
Lastly, nutritional malabsorption can also present with dermatologic manifestations, as described in Table 2. This is not a common diagnosis but is something to keep in mind.
Conclusions
In summary, there are a range of dermatological extra-intestinal manifestations of IBD including specific and reactive manifestations, associated disorders, and treatment-related phenomena. An important consideration is the psoriasiform eruptions induced by anti-TNFs, which often require a treatment switch. Newer targeted therapies may play a role in the management of dermatologic manifestations, and continued collaboration between specialties is important to improve outcomes.
Clinical Case
Jacob—19-year-old patient with prior history of:
- Childhood asthma
- Small perianal abscess at age 12 that healed after local incision and drainage
Presented recently to family doctor with firm red tender nodules on both shins
- Treated twice with prednisone for presumed erythema nodosum
Shortly after second course of prednisone, while awaiting dermatology consultation, he reported:
- Blood in his stool
- Increased stool frequency
Urgent colonoscopy by a local surgeon at ambulatory clinic found:
- Serpiginous ulceration
- Cobblestoning in the sigmoid and ascending colon
Ileum was not intubated
Biopsies consistent with Crohn’s disease
You see him in urgent consultation
Commentary
Biopsy can be helpful to help differentiate the underlying cause of psoriasiform eruptions.
In terms of treatment selection in this case, most gastroenterologists would choose an anti-TNF, specifically infliximab.
Case Evolution
Jacob starts on an infliximab biosimilar
- Excellent response with respect to his gastrointestinal symptoms
- No relapse of his erythema nodosum
He did not attend his dermatology appointment
Colonoscopy after 8 months shows:
- Ileal aphthous ulcers
- A few sigmoid aphthous ulcers
- Small inflammatory polyps throughout the colon
In the recovery room, he asks you to look at his rash
He points to raised and scaling patches around his nose, behind his ears, and on his scalp
You suspect a psoriasiform skin eruption induced by his anti-TNF therapy
You send a new dermatology referral, but there is a 6-month wait
Jacob is adamant that he needs something to be done now
Commentary
For the next step for this patient, most gastroenterologists would continue infliximab and treat the rash topically, while some would consider switching to a different mechanism of action, e.g., risankizumab.
- It was noted that a new non-steroidal topical option is now available for psoriasis, roflumilast (a PDE4 inhibitor), that can even be used on the face.
In practice, some gastroenterologists have a connection with a dermatologist, so can reach out rapidly, and given their limited comfort with managing dermatologic manifestations, they try to develop collaborations whenever possible.
Ideally, they would like to receive a rapid consult or be provided with direction on what therapy to start while waiting for a full consult.
Patients do not know psoriasis can be related to their IBD treatment, so it is important for gastroenterologists to educate patients to inform them about these things.
They find that most of their patients are so sick they will accept some psoriasis to have improvement in their IBD—discussion around dermatologic manifestations tends to come up later.
Case Evolution
You switch him to ustekinumab as he likes the idea of fewer injections and infusions
His skin improves
His gastrointestinal symptoms remain controlled
His dermatologist agrees with your treatment choice
After a year on therapy, his Crohn’s disease remains well controlled, but…
He returns to his dermatologist complaining that the rash has returned
This time, a small nodule on his left shin has evolved into an open sore
His dermatologist diagnoses pyoderma gangrenosum and prescribes a course of prednisone
Unfortunately, Jacob has only partial healing
Dermatologist calls you to discuss options for advanced therapy
Commentary
Although PG is less common than other dermatologic manifestations, it is often more severe, and may not be associated with intestinal disease activity.
For treatment modifications at this point in the case, most gastroenterologists would increase the ustekinumab dose, while some would switch to risankizumab or add adalimumab.
It was noted that the three IL-23 inhibitors currently available differ in their potency—something dermatologists consider when choosing within the class.
Case Evolution
You decide to continue his ustekinumab and add biosimilar adalimumab
Again, he does well with respect to his gastrointestinal symptoms
The pyoderma heals with only residual scarring
Commentary
For next steps in this case, most gastroenterologists would stop ustekinumab and continue adalimumab monotherapy, while some would stop adalimumab and continue ustekinumab.
The consensus was that some therapy de-escalation can be considered at this point but there is reluctance to completely stop therapy due to the risk of his bowel disease recurring.
References
- Lipson, J. Cutaneous manifestations of inflammatory bowel disease. Can IBD Today. 2023;1(2):17–23.
- Antonelli E, Bassotti G, Tramontana M, et al. Dermatological manifestations in inflammatory bowel diseases. J Clin Med. 2021:10(2):364.
- Yüksel I, Basar O, Ataseven H, et al. Mucocutaneous manifestations in inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:546–50.
- Alignaghi F, Göcker Tekin H, Burisch J, et al. Global prevalence and bidirectional association between psoriasis and inflammatory bowel disease-a systematic review and meta-analysis. J Crohn’s Colitis. 2020;14(3):351–60.
- Chen W-T, Chi C-C. Association of hidradenitis suppurativa with inflammatory bowel disease: A systematic review and meta-analysis. JAMA Dermatol. 2019;155(9):1022–7.
- Yousif M, Ritchey A, Mirea L, et al. Erythema nodosum and pyoderma gangrenosum in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2023; 29 (Supplement1): S60–S1. (Abstract)
- States V, O’Brien S, Rai JP, et al. Pyoderma gangrenosum in inflammatory bowel disease: A systematic review and meta-analysis. Dig Dis Sci. 2020;65:2675–85.
- Xie W, Xiao S, Huang H, et al. Incidence of and risk factors for paradoxical psoriasis or psoriasiform lesions in inflammatory bowel disease patients receiving anti-TNF therapy: Systematic review with meta-analysis. Front Immunol. 2022:13:847160.
- Li SJ, Perez-Chada LM, Merola JF. TNF inhibitor-induced psoriasis: Proposed algorithm for treatment and management. J Psoriasis Psoriatic Arth. 2019;4:70–80.
- Eickstaedt J, Paller AS, Lund E, et al. Paradoxical psoriasiform eruptions in children receiving tumor necrosis factor inhibitors. JAMA Dermatol. 2023;159(6):637–42.
- Dolinger MT, Rolfes P, Spencer E, et al. Outcomes of children with inflammatory bowel disease who develop anti-tumour necrosis factor-induced skin reactions. J Crohn’s Colitis. 2022;16:1420–7.
- Livne-Margolin M, Ling D, Attia-Konyo S, et al. Ustekinumab and vedolizumab for extraintestinal manifestations in inflammatory bowel disease – a retrospective study. Dig Liver Dis. 55;2023:223–9.
Editor-in-Chief
John K. Marshall, MD MSc FRCPC CAGF AGAF
Professor, Department of Medicine
Director, Division of Gastroenterology
McMaster University
Hamilton, ON
Contributing Author
Melinda GooderhamMD MSc FRCPC
Assistant Professor, Queen’s University
Consultant Physician, Peterborough Regional Health Centre (PRHC)
Medical Director, SKiN Centre for Dermatology
Principal Investigator, SKiN Research Centre
Peterborough, ON
Mentoring in IBD Curriculum Steering Committee
Alain Bitton, MD FRCPC, McGill University, Montreal, QC
Karen I. Kroeker, MD MSc FRCPC, University of Alberta, Edmonton, AB
Cynthia Seow, MBBS (Hons) MSc FRACP, University of Calgary, Calgary, AB
Jennifer Stretton, ACNP MN BScN, St. Joseph’s Healthcare, Hamilton, ON
Eytan Wine, MD PhD, FRCPC, University of Alberta, Edmonton, AB
IBD Dialogue 2024·Volume 20 is made possible by unrestricted educational grants from…
![]()
![]()







Published by Catrile & Associates Ltd., 167 Floyd Avenue, East York, ON M4J 2H9
(c) Catrile & Associates Ltd., 2024. All rights reserved. None of the contents may be reproduced in any form without prior written permission from the publisher. The opinions expressed in this paper are those of the authors and do not necessarily reflect the opinions or recommendations of the sponsors, the grantor, or the publisher.